44 phase labels in chemical equations
The Chemical Equation - Introductory Chemistry - 1st Canadian Edition Write and balance the chemical equation that represents nitrogen and hydrogen reacting to produce ammonia, NH 3. Answer N 2 + 3H 2 → 2NH 3 Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water). Chemical Equations - GitHub Pages It is not uncommon to include a phase label with each formula— (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for a substance dissolved in water, also known as an aqueous solution. If we included phase labels for the reactants and products, under normal environmental conditions, the reaction would be as follows: H2(g) + O2(g) → H2O (ℓ) Note
Solved 1. Write a balanced thermochemical equation with | Chegg.com Question: 1. Write a balanced thermochemical equation with phase labels for the Haber process with the heat energy as part of the equation 2. What is the theoretical yield of ammonia (in grams) if 16.55 grams of nitrogen gas and 10.15 grams of hydrogen gas are allowed to react? 3.
Phase labels in chemical equations
chemical equation | Yeah Chemistry Formulas of the reactants appear on the left side of the equation; those of products are written on the right. In many cases it is useful to indicate the state or phases of the substance in an equation.You use the following phase labels: (g) = gas , (l) = liquid , (s) = solid , (aq) = water solution End-of-Chapter Material | Introductory Chemistry - Lumen Learning Chemical equations can also be used to represent physical processes. Write a chemical reaction for the freezing of water, including the proper phase labels. Explain why 4 Na (s) + 2 Cl2(g) → 4 NaCl (s) should not be considered a proper chemical equation. Explain why H2(g) + 1/2 O2(g) → H2O (ℓ) should not be considered a proper chemical equation. State symbols and phase changes - StudyPug The phase can affect how reactive a substance is, but changing phase (a physical change) is not the same as changing the substance (a chemical change). A fully detailed chemical equation will show the state (or phase) of matter that the atoms or molecules are in. These states are: Solid, given the symbol (s) Liquid, given the symbol (l)
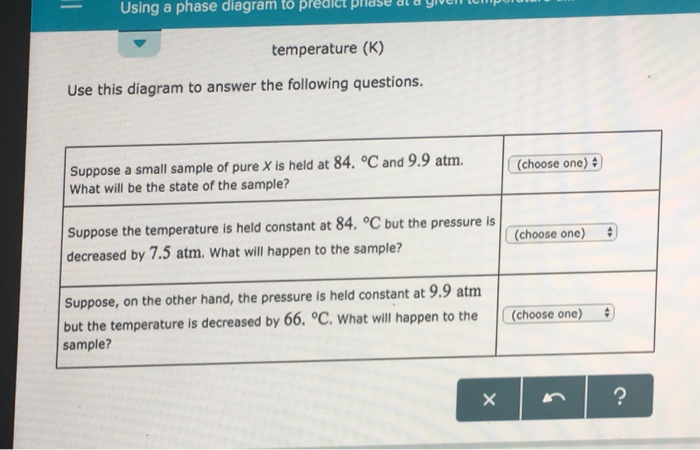
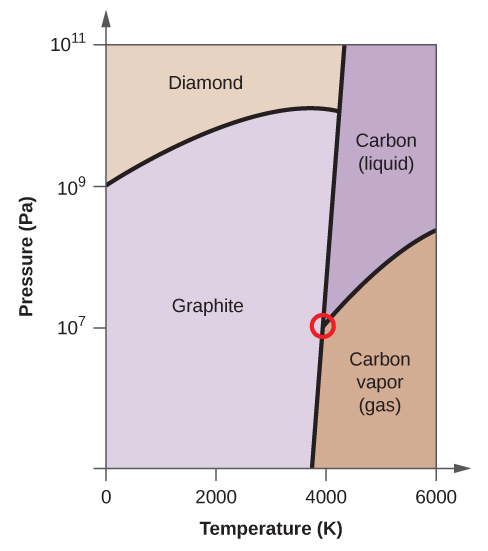
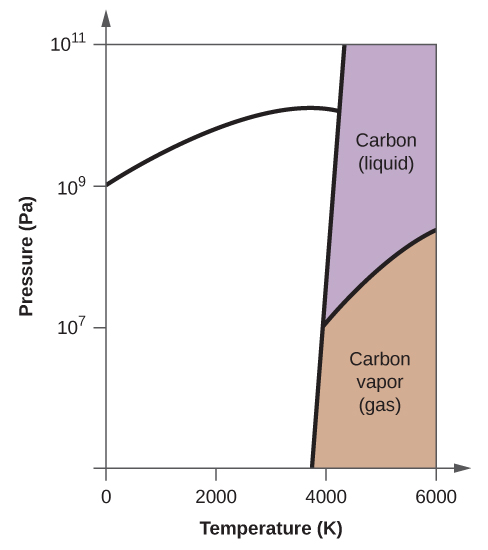
Phase labels in chemical equations. Phase Diagrams - Chemistry - University of Hawaiʻi (a) On the phase diagram, label the gas and liquid regions. (b) Graphite is the most stable phase of carbon at normal conditions. On the phase diagram, label the graphite phase. (c) If graphite at normal conditions is heated to 2500 K while the pressure is increased to 10 10 Pa, it is converted into diamond. Label the diamond phase. Phase Definition and Examples - ThoughtCo A phase of matter is characterized by having relatively uniform chemical and physical properties. Phases are different from states of matter. The states of matter (e.g., liquid, solid, gas) are phases, but matter can exist in different phases yet remain in the same state of matter. For example, liquid mixtures can exist in multiple phases, such ... Conventions for Writing Chemical Equations - Arrows, Phases ... - YouTube presents: Conventions for Writing Chemical Equations including Arrows, Phases, Coefficients, Reactants, Products and moreTired... 3 Steps for Balancing Chemical Equations - ThoughtCo A chemical equation describes what happens in a chemical reaction.The equation identifies the reactants (starting materials) and products (resulting substances), the formulas of the participants, the phases of the participants (solid, liquid, gas), the direction of the chemical reaction, and the amount of each substance. Chemical equations are balanced for mass and charge, meaning the number ...
End-of-Chapter Material - Introductory Chemistry - 1st Canadian Edition Chemical equations can also be used to represent physical processes. Write a chemical reaction for the freezing of water, including the proper phase labels. Explain why 4Na (s) + 2Cl 2 (g) → 4NaCl (s) should not be considered a proper chemical equation. Explain why H 2 (g) + ½O 2 (g) → H 2 O (ℓ) should not be considered a proper chemical equation. End-of-Chapter Material - GitHub Pages Chemical equations can also be used to represent physical processes. Write a chemical reaction for the freezing of water, including the proper phase labels. Explain why 4Na (s) + 2Cl2(g) → 4NaCl (s) should not be considered a proper chemical equation. Explain why H2(g) + 1/2O2(g) → H2O (ℓ) should not be considered a proper chemical equation. EXAM #2: Chapter 4 Flashcards | Quizlet Write a chemical reaction for the boiling of water, including the proper phase labels. ... 2. Chemical equations can also be used to represent physical processes. Write a chemical reaction for the freezing of water, including the proper phase labels. ... 3. Explain why 4Na (s)+2ClX2 (g) 4NaCl (s) should not be considered a proper chemical equation. End-of-Chapter Material - Introductory Chemistry- 1st Canadian Edition Chemical equations can also be used to represent physical processes. Write a chemical reaction for the freezing of water, including the proper phase labels. Explain why 4 Na (s) + 2 Cl2(g) → 4 NaCl (s) should not be considered a proper chemical equation. Explain why H2(g) + 1/2 O2(g) → H2O (ℓ) should not be considered a proper chemical equation.
Phase Diagram | Explanation, Definition, Summary & Facts A phase has a complete distinct physical and chemical properties, whereas two phases are separated by a phase boundary. A phase diagram is a graphical representation of the substance phases, consists of the curved lines and the space between the two lines represent a specific phase of the matter at given pressure and temperature, whereas any ... Chapter 5 - Chemical Reactions and Equations - CHE 105/110 ... Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water). Special conditions, such as temperature, may also be listed above the arrow. For example, 2NaHCO3 (s) − →−200°C Na2CO3 (s) +CO2(g) +H2O(ℓ) Key Takeaways 5.2 Chemical Equations - Lumen Learning It is not uncommon to include a phase label with each formula— (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for a substance dissolved in water, also known as an aqueous solution. If we included phase labels for the reactants and products, under normal environmental conditions, the reaction would be as follows: H2(g) + O2(g) → H2O (ℓ) Note 5.7 End-of-Chapter Material - Introductory Chemistry - 1st Canadian ... Write a chemical reaction for the boiling of water, including the proper phase labels. Chemical equations can also be used to represent physical processes. Write a chemical reaction for the freezing of water, including the proper phase labels. Explain why. 4 Na(s) + 2 Cl 2 (g) → 4 NaCl(s)
Solved 1. Write balanced chemical equations using proper | Chegg.com 1. Write balanced chemical equations using proper phase labels for these reactions: (you may also handwrite in the equations) a) Iron metal with oxygen gas to produce solid iron (III) oxide, also known as rust.
Writing and Balancing Chemical Equations - Chemistry 2e 1 2 = 2. 2 = 2, yes. These coefficients yield equal numbers of both H and O atoms on the reactant and product sides, and the balanced equation is, therefore: Balancing Chemical Equations Write a balanced equation for the reaction of molecular nitrogen (N 2) and oxygen (O 2) to form dinitrogen pentoxide. Solution First, write the unbalanced ...
Phase Transitions: Melting, Boiling, and Subliming Chemical equations can be used to represent a phase change. In such cases, it is crucial to use phase labels on the substances. For example, the chemical equation for the melting of ice to make liquid water is as follows: H 2 O (s) → H 2 O (ℓ) No chemical change is taking place; however, a physical change is taking place. Heating Curves
A thermochemical equation is a balanced chemical reaction equation ... An equation which shows both mass and heat relationships between products and reactants is called a thermochemical equation. The following four reactions are examples of thermochemical equations. The first two are exothermic and the last two are endothermic reactions. 2 H 2 (g) + O 2 (g) ----> 2 H 2 O(l) DH = -571.6 kJ
4.1 Writing and Balancing Chemical Equations - Chemistry 10.3 Phase Transitions. 10.4 Phase Diagrams. 10.5 The Solid State of Matter. 10.6 Lattice Structures in Crystalline Solids. Chapter 11. Solutions and Colloids ... change involves writing and balancing a chemical equation. Consider as an example the reaction between one methane molecule (CH 4) and two diatomic oxygen molecules (O 2) to produce ...
The Chemical Equation - GitHub Pages Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water). Special conditions, such as temperature, may also be listed above the arrow. For example, Key Takeaways A chemical equation is a concise description of a chemical reaction.
Solved Part 7: Aqueous Chemistry...Ionic Equations 7. In | Chegg.com In many aqueous chemical reactions, there are ions that are not involved in the chemical change but serve to deliver the ions that are involved. hen a compound in a chemical equation has an aqueous label, and is ionic, it consists of the constituent ions separated in solution. We represent the ions in a complete This question hasn't been solved yet
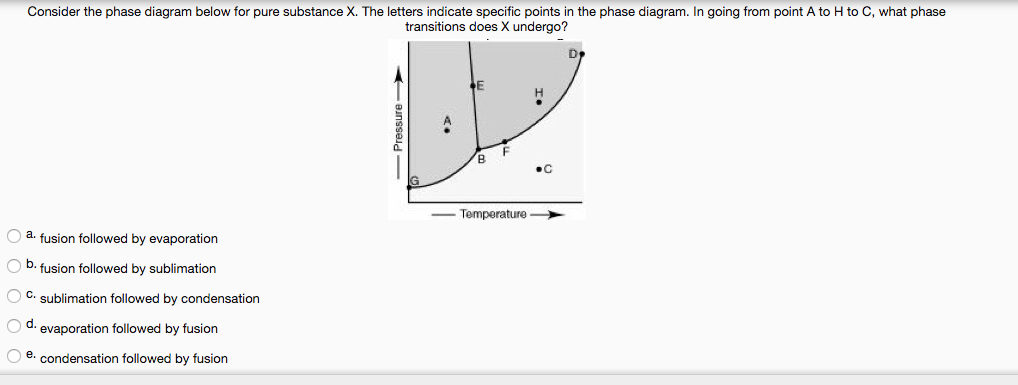
Phase diagram - Wikipedia A phase diagram in physical chemistry, engineering, mineralogy, and materials science is a type of chart used to show conditions (pressure, temperature, volume, etc.) at which thermodynamically distinct phases (such as solid, liquid or gaseous states) occur and coexist at equilibrium . Contents 1 Overview 2 Types 2.1 2-dimensional diagrams
Solved 1. Write a balanced thermochemical equation with | Chegg.com Question: 1. Write a balanced thermochemical equation with phase labels for the Haber process with the heat energy as part of the equation. (3 pts) 2. What is the theoretical yield of ammonia (in grams) if 16.55 grams of nitrogen gas and 10.15 grams of hydrogen gas are allowed to react? (9 pts) 3.
State symbols and phase changes - StudyPug The phase can affect how reactive a substance is, but changing phase (a physical change) is not the same as changing the substance (a chemical change). A fully detailed chemical equation will show the state (or phase) of matter that the atoms or molecules are in. These states are: Solid, given the symbol (s) Liquid, given the symbol (l)
End-of-Chapter Material | Introductory Chemistry - Lumen Learning Chemical equations can also be used to represent physical processes. Write a chemical reaction for the freezing of water, including the proper phase labels. Explain why 4 Na (s) + 2 Cl2(g) → 4 NaCl (s) should not be considered a proper chemical equation. Explain why H2(g) + 1/2 O2(g) → H2O (ℓ) should not be considered a proper chemical equation.
chemical equation | Yeah Chemistry Formulas of the reactants appear on the left side of the equation; those of products are written on the right. In many cases it is useful to indicate the state or phases of the substance in an equation.You use the following phase labels: (g) = gas , (l) = liquid , (s) = solid , (aq) = water solution







.png)




Post a Comment for "44 phase labels in chemical equations"